Pharma
AbbVie in Free Fall: Billion-Dollar Investment in Schizophrenia Medication Falls Short of Expectations
Pharma giant AbbVie suffers severe setback – new schizophrenia drug fails in clinical trial, while competitors triumph
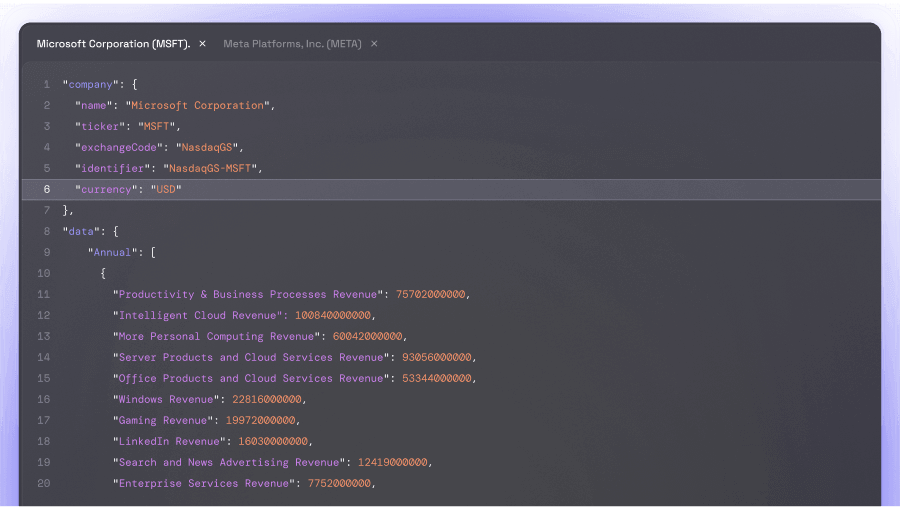
The news came like a bombshell and sent AbbVie's stock prices plummeting: The new schizophrenia drug Emraclidine, which the US pharmaceutical giant had invested $8.7 billion in just last year, fell significantly short of expectations in a Phase II clinical trial. A severe setback for AbbVie and its investors – and an unexpected gain for the competition.
Clinical Study Disappoints: Emraclidine Without Significant Effect
On Monday, AbbVie announced that the drug Emraclidine, tested in the study, failed to show a "statistically significant reduction" in psychotic symptoms for schizophrenia patients. The study involved 752 patients, and despite high expectations, the difference from the placebo group was absent. Within a short time, the stock plummeted by more than 12 percent, and AbbVie lost over 40 billion dollars in market value – a bitter moment for a company that had set high goals in the field of psychiatry.
Emraclidine was the central component of AbbVie's strategy to compete with the major players in the field of schizophrenia medications after the purchase of Cerevel Therapeutics for $8.7 billion. AbbVie particularly targeted Bristol Myers Squibb (BMS), whose new drug Cobenfy was only approved as a groundbreaking therapy for schizophrenia in September. But now, this goal seems to have become distant.
Bristol Myers Squibb benefits from AbbVie's setback
While AbbVie lies in shambles, BMS can be pleased about growing investor approval.
Psychiatry as a Tough Nut for the Pharmaceutical Industry
For years, psychiatry has been considered one of the most difficult and risky fields for the pharmaceutical industry. The complexity of the human brain and the often unreliable animal models make it challenging to develop effective medications. Therefore, many companies have completely abandoned the field. However, the approval of Cobenfy in September had raised hopes that Emraclidine could become the next groundbreaking drug for schizophrenia.
An analyst from BMO Capital Markets, Evan Seigerman, commented that the failure of the study represents a "difficult outcome" for AbbVie. And although another drug, acquired as part of the Cerevel deal, was successful in late-stage studies for Parkinson's diseases, "today's result casts a poor light on the $8.7 billion deal.
Doubts About AbbVie's Strategy Are Growing
Here's the translation of the heading to English:
"Even analysts like Vamil Divan from Guggenheim Securities expressed skepticism about AbbVie's long-term strategy. With the potential loss of $1.5 billion in revenue from Emraclidine by 2033, the strategy could prove to be overstretched. Although AbbVie remains free from major patent expirations until 2029, doubts about the company's ability to generate additional growth momentum are increasing.
I can provide a translation for the given text, but it seems you've included more than a heading. Here is the translation for the entire segment:
"AbbVie's Chief Scientific Officer Roopal Thakkar expressed disappointment over the result and emphasized that the company would continue to analyze the data to identify possible next steps. 'We are confident that our innovative pipeline will continue to produce significant therapies for patients, and we remain committed to finding better treatments for people with psychiatric and neurological disorders,' said Thakkar.
The translation of the heading to English is:
"Whether AbbVie can turn this confidence into real success remains to be seen. Meanwhile, the pharmaceutical world is eagerly watching the next steps of the industry in schizophrenia treatment, where the competition currently appears to be significantly superior.